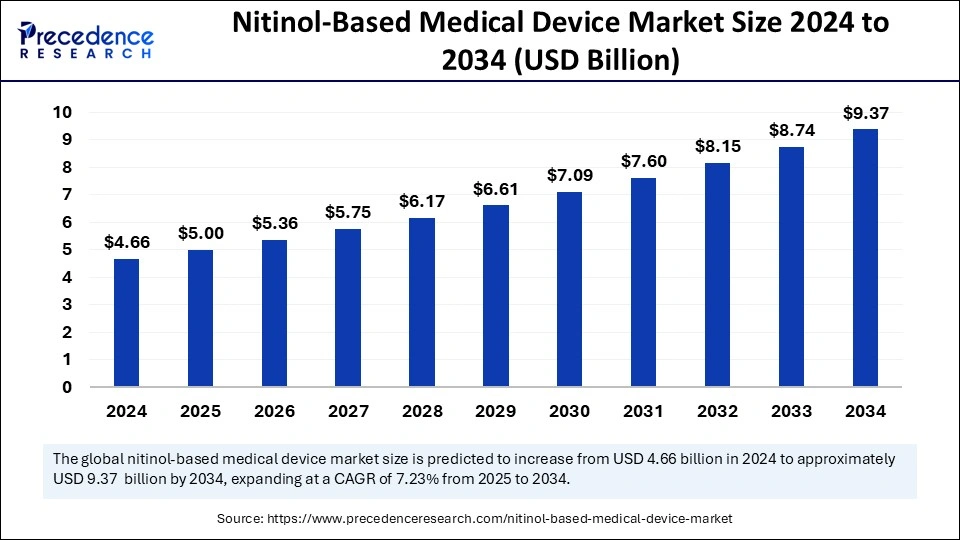
Nitinol-Based Medical Device Market Key Takeaways
- North America dominated the nitinol-based medical device market in 2024.
- Asia Pacific is expected to grow at a CAGR of 8.06% between 2025 and 2034.
- By product type, the retriever device segment dominated the market in 2024.
- By product type, the catheters segment is expected to grow at the highest CAGR during the projection period.
- By application, the cardiovascular segment led the market in 2024.
- By end-use, the hospitals segment held the dominant market share in 2024.
- By end-use, the ambulatory surgical centers segment is projected to grow at a CAGR of 8.1% between 2025 and 2034.
Nitinol-Based Medical Device Market Overview
The nitinol-based medical device market is witnessing rapid growth due to the rising advancements in nitinol processing and manufacturing technologies. Advancements in manufacturing technologies led to the development of more sophisticated nitinol-based medical devices. Being a metallic biomaterial and having unique properties such as superelasticity, shape memory, and greater damping characteristics make nitinol a preferred material for use in medical devices. It is a very useful material in orthopedic implants and other surgical instruments.
Despite the greater initial nickel dissolution, it does not induce toxic effects, reduce cell proliferation, or prevent the growth of cells in contact with the metal surface. Nitinol is potentially used in cardiac devices like vena cava filters, stents, guidewires, and heart valves. The applications of nitinol in other equipment areas are rising, predominantly for products intended for usage in minimally invasive procedures. Nitinol is a well-known and common engineering material in the healthcare sector.
Nitinol-Based Medical Device Market Drivers
The primary drivers of this market include the rising prevalence of cardiovascular diseases and the growing aging population, which necessitates advanced medical interventions. The unique properties of Nitinol allow for the development of devices that can adapt to the dynamic environment of the human body, enhancing patient outcomes. Additionally, the shift towards minimally invasive surgical procedures has propelled the adoption of Nitinol-based devices due to their flexibility and biocompatibility.
Nitinol-Based Medical Device Market Opportunities
Emerging markets present significant opportunities for the expansion of Nitinol-based medical devices. The increasing healthcare expenditure and improving infrastructure in regions like Asia-Pacific and Latin America are creating a conducive environment for market growth. Furthermore, ongoing research and development activities aimed at enhancing the properties of Nitinol and expanding its applications in areas such as neurology and urology are expected to open new avenues for market players.
Nitinol-Based Medical Device Market Challenges
Despite the promising growth prospects, the market faces challenges such as the high cost of Nitinol-based devices and the complexity of manufacturing processes. Additionally, stringent regulatory requirements and the need for extensive clinical trials can delay product approvals and market entry. The potential for nickel-related allergic reactions also necessitates thorough biocompatibility assessments, adding to the development timeline and costs.
Nitinol-Based Medical Device Market Regional Insights
North America currently dominates the Nitinol-based medical device market, accounting for a significant share due to advanced healthcare infrastructure, high adoption rates of innovative technologies, and the presence of leading medical device manufacturers. Europe follows closely, with countries like Germany, France, and the UK investing heavily in healthcare innovation. The Asia-Pacific region is anticipated to witness the highest growth rate, driven by increasing healthcare awareness, rising disposable incomes, and government initiatives to improve healthcare access.
Nitinol-Based Medical Device Market Recent Developments
Recent developments in the market include strategic collaborations and acquisitions aimed at expanding product portfolios and market reach. For instance, in January 2023, Confluent Medical Technologies announced a partnership with medical device OEMs and secured an investment from TPG Capital to accelerate the development of its biocompatible Nitinol-based vascular closure devices. Additionally, advancements in manufacturing technologies are enabling the production of more complex and reliable Nitinol-based devices, further propelling market growth.
Nitinol-Based Medical Device Market Companies
- Medtronic plc
- Terumo Corporation
- Cordis
- Zimmer Biomet Holding, Inc.
- Becton, Dickinson, and Company
- W.L. Gore & Associates Inc
- Boston Scientific Corporation
- Arthrex Inc
- Cook Group
- Solventum
Segments Covered in the Report
By Product Type
- Stents
- Guidewires
- Retriever Device
- Catheters
- Others
By Application
- Dentistry
- Urology
- Others
- Cardiovascular
By End-use
- Hospitals
- Ambulatory Surgical Centers
- Others
By Region
- North America
- Europe
- Asia Pacific
- Middle East & Africa
- Latin America
Ready for more? Dive into the full experience on our website!