Immunology Biosimilars Market Key Takeaways
- In 2024, North America held the leading position in the immunology biosimilars market.
- Asia Pacific is expected to be the fastest-growing region throughout the forecast duration.
- Europe continues to emerge as a key region in the evolving market landscape.
- Inflammatory bowel disease was the most dominant disease segment in 2024.
- Arthritis is likely to register the most accelerated growth during the forecasted timeframe.
- Hospital pharmacies dominated the distribution segment by market share in 2024.
- The retail pharmacies channel is projected to see substantial growth moving forward.
Market Overview
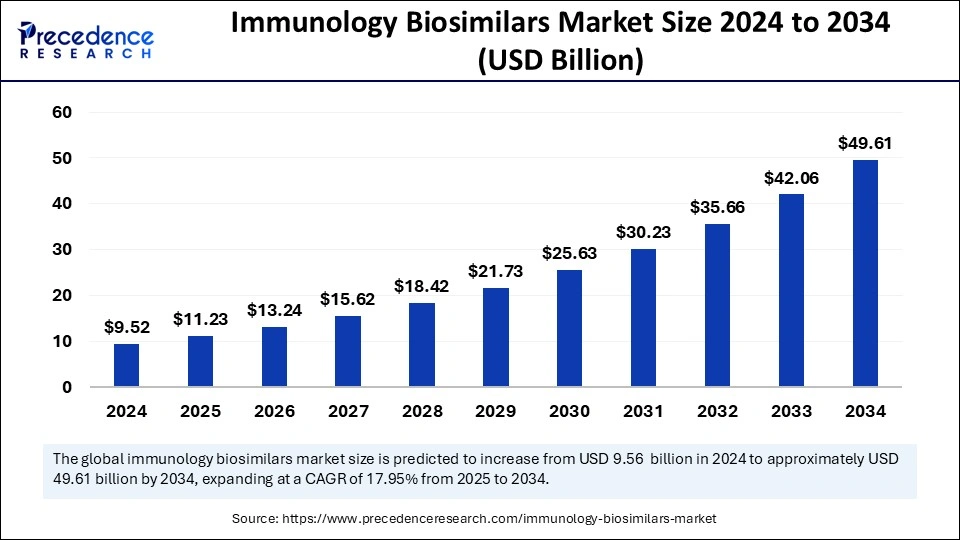
The immunology biosimilars market is at the center of a transformation within biopharmaceuticals, aiming to deliver cost-effective, high-quality therapeutic alternatives to biologic drugs. Biosimilars are increasingly being adopted across various immunological conditions, helping reduce the economic burden of chronic autoimmune disorders. As biologic drug patents expire, biosimilars are stepping in to meet the therapeutic needs of a growing global population.
The immunology biosimilars market has gained momentum due to the ability of biosimilars to closely mirror the originator biologic in terms of clinical outcomes. This therapeutic parity, along with cost benefits, is encouraging healthcare systems, providers, and patients to shift toward biosimilars. Rising demand for value-based healthcare and drug affordability is catalyzing the adoption of biosimilars, contributing to the expansion of the immunology biosimilars market.
Moreover, as pharmaceutical companies strive to balance innovation and accessibility, biosimilars offer a sustainable business model. Governments and healthcare payers are actively promoting biosimilar uptake, resulting in an expanding presence of the immunology biosimilars market across both high- and low-income regions.
Drivers
A major growth driver of the immunology biosimilars market is increasing global healthcare expenditure. With chronic immune conditions placing substantial financial pressure on healthcare systems, biosimilars present a practical solution. Their lower price points allow broader access while maintaining therapeutic efficacy, which in turn fuels market growth.
The increasing number of biologic patent expirations is another driving force. As the exclusivity period of top-selling biologics comes to an end, the immunology biosimilars market opens up for competition. This trend is expected to continue over the next decade, offering long-term growth opportunities for biosimilar developers.
The support of healthcare professionals is also growing. Clinicians are more confident in prescribing biosimilars as clinical trials and post-marketing surveillance data reinforce safety and efficacy. This trust is vital in encouraging the widespread acceptance of the immunology biosimilars market.
Opportunities
One of the most promising opportunities in the immunology biosimilars market is market penetration in underserved areas. Countries with limited access to biologics can greatly benefit from biosimilars, which are more affordable and increasingly available due to improving distribution networks.
There is also growing interest in the development of interchangeable biosimilars, which can be substituted without physician intervention. If more biosimilars achieve this designation, the immunology biosimilars market could experience a surge in prescriptions, particularly at the pharmacy level.
Moreover, technological innovations are opening new doors. From improved purification processes to AI-assisted clinical trial design, technology is enhancing biosimilar development efficiency. Firms that embrace these innovations are better positioned to lead the immunology biosimilars market.
Challenges
Despite encouraging trends, the immunology biosimilars market faces regulatory, scientific, and commercial barriers. Biosimilar development requires a high level of expertise and significant investment, which may deter smaller firms.
The lack of standardization in global regulatory frameworks is also a concern. While regions like the EU and US have mature systems, other markets are still refining their approval pathways. These discrepancies can delay product launches and affect the pace of global expansion in the immunology biosimilars market.
Resistance from originator companies remains another issue. Through patent litigation and strategic pricing, innovators attempt to defend their market share, sometimes stalling biosimilar adoption. This is a persistent challenge in the immunology biosimilars market, necessitating policy reforms and market education.
Regional Insights
The immunology biosimilars market is geographically diverse, with distinct dynamics in each region. In North America, growing acceptance and supportive reimbursement models are helping biosimilars gain traction. Market players are focusing on education and partnerships with healthcare providers to accelerate adoption.
Europe continues to lead in biosimilar utilization, driven by the early regulatory leadership of the EMA. The region has achieved substantial cost savings and expanded access through biosimilar integration. Countries like the UK and Germany have demonstrated best practices in biosimilar substitution, benefiting the immunology biosimilars market.
Asia-Pacific is gaining prominence due to population size and government support for biosimilars. Countries such as China and India are expanding their domestic biosimilar capabilities, boosting both production and consumption in the immunology biosimilars market.
In Latin America and Africa, while regulatory hurdles persist, improving healthcare frameworks and international collaboration are paving the way for future growth. These regions are expected to contribute meaningfully to the global immunology biosimilars market in the next decade.
Recent Developments
The immunology biosimilars market has seen a wave of strategic launches and innovations. Several biosimilars for immunology biologics have received regulatory approval in the last few years, expanding therapeutic options for conditions like rheumatoid arthritis and inflammatory bowel disease.
Strategic alliances are on the rise. Companies are entering partnerships to co-develop biosimilars, share risk, and leverage market reach. These collaborations are expected to drive pipeline development in the immunology biosimilars market.
Immunology Biosimilars Market Companies
- Novartis AG
- Pfizer Inc.
- Mylan N.V.
- AbbVie Inc.
- STADA Arzneimittel AG
- Celltrion, Inc.
- KBI Biopharma, Inc.
- Amgen, Inc
Segments Covered in the Report
By Disease
- Inflammatory Bowel Disease
- Arthritis
- Others
By Distribution Channel
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
- Others
By Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa
Ready for more? Dive into the full experience on our website!