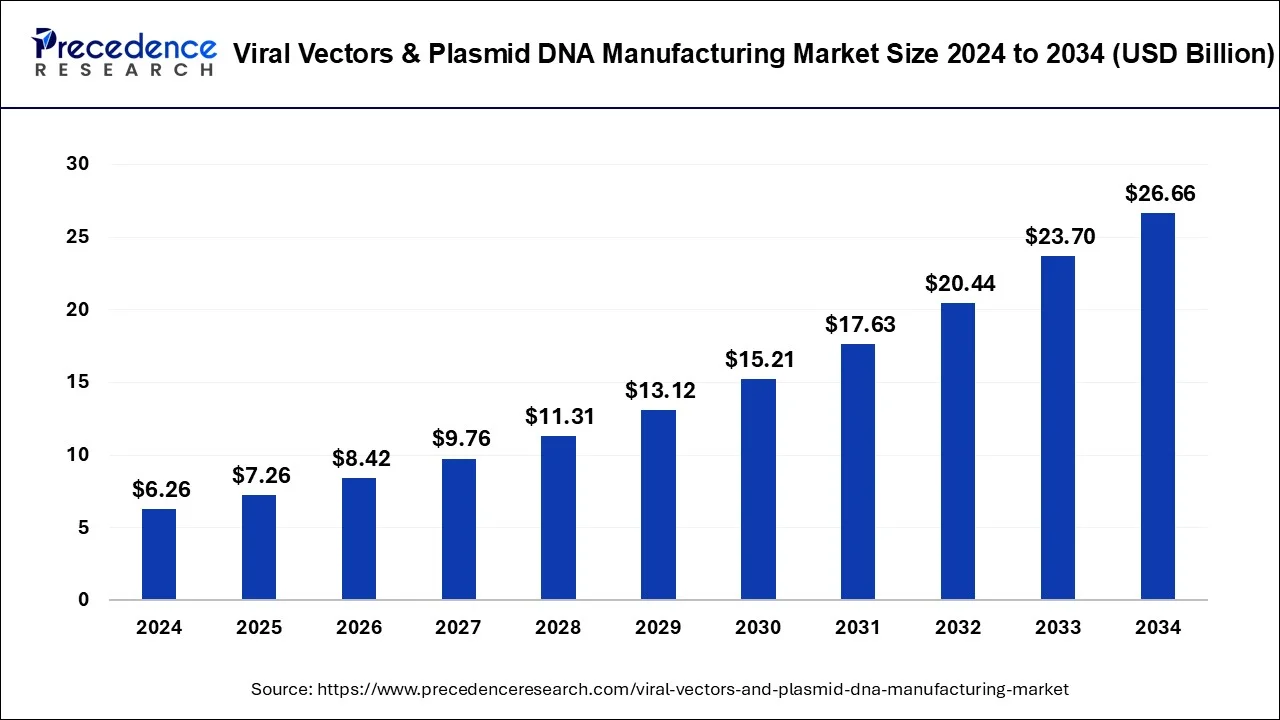
The viral vectors & plasmid DNA manufacturing market is expected to grow from USD 6.26 billion in 2024 to USD 26.66 billion by 2034.
Viral Vectors & Plasmid DNA Manufacturing Market Key Takeaways
- In 2024, North America maintained its dominance in the viral vectors & plasmid DNA manufacturing market with a 49% revenue share.
- The AAV vector type led the market, contributing 21% of total revenue in 2024.
- The downstream processing segment held a leading position in the workflow category with 54% market share in 2023.
- The vaccinology segment was the top application, accounting for 22.5% of market revenue in 2024.
- Cancer treatment applications drove market growth, with a 38% revenue share in 2024.
- Research institutes remained the primary end-user, capturing 58.4% of the market share in 2024.
Market Overview
The viral vectors and plasmid DNA manufacturing market is experiencing rapid growth, driven by the rising demand for gene therapies, vaccines, and cell-based research. These genetic materials are essential for gene transfer, enabling the development of innovative treatments for genetic disorders, cancer, and infectious diseases. Advances in biotechnology, coupled with the expanding pipeline of gene therapies and personalized medicine, are fueling the need for large-scale, high-quality production. As biopharmaceutical companies and research institutions continue to innovate and develop advanced therapeutics, the market is set for significant expansion in the coming years.
Market Drivers
Several factors are fueling the growth of the viral vectors and plasmid DNA manufacturing market. The rising prevalence of genetic and chronic diseases has intensified the demand for gene therapies and advanced biologics, requiring efficient manufacturing processes. The COVID-19 pandemic highlighted the importance of viral vector-based vaccines, accelerating research and development efforts in this field. Additionally, the increasing number of clinical trials focused on cell and gene therapies has created a strong demand for viral vector and plasmid DNA production. Government initiatives and funding support for gene therapy research, along with technological advancements in bioprocessing and manufacturing scalability, are further propelling market growth.
Opportunities in the Market
The market presents numerous opportunities, particularly in the expansion of contract development and manufacturing organizations (CDMOs) that provide scalable and cost-effective manufacturing solutions. As demand for viral vectors and plasmid DNA grows, companies specializing in large-scale production and process optimization are gaining traction. The emergence of non-viral delivery systems and advancements in synthetic biology offer additional avenues for innovation. Moreover, the increasing collaborations between biopharmaceutical companies, research institutions, and regulatory bodies are fostering advancements in manufacturing technologies, ensuring the efficient production of high-quality gene therapy products.
Challenges Facing the Market
Despite its growth potential, the viral vectors and plasmid DNA manufacturing market faces several challenges. High production costs and complex manufacturing processes pose significant barriers to market expansion. The stringent regulatory requirements for gene therapy products necessitate rigorous quality control measures, which can slow down production timelines and increase operational costs. Additionally, the limited availability of skilled professionals in bioprocessing and gene therapy manufacturing creates workforce challenges for companies. The scalability of manufacturing processes remains a critical issue, as demand for gene therapies continues to rise, requiring enhanced production capabilities and infrastructure investments.
Regional Insights
The market for viral vectors and plasmid DNA manufacturing exhibits strong growth across various regions. North America dominates the market, driven by a well-established biotechnology sector, extensive research and development activities, and strong government support for gene therapy innovations. The United States, in particular, leads in clinical trials and regulatory approvals for gene-based treatments. Europe is also a significant player, with countries such as Germany, the United Kingdom, and France investing heavily in biopharmaceutical research and manufacturing.
The Asia-Pacific region is emerging as a lucrative market due to increasing biopharmaceutical investments, expanding healthcare infrastructure, and a growing number of biotechnology startups in countries like China, Japan, and South Korea. Meanwhile, Latin America and the Middle East are gradually entering the market, with rising interest in advanced therapeutics and vaccine development.
Recent Developments
The viral vectors and plasmid DNA manufacturing market has experienced significant advancements in recent months. Biopharmaceutical companies are scaling up their manufacturing facilities to accommodate the increasing demand for gene therapy products. Investments in automation and advanced bioprocessing technologies are improving production efficiency and scalability. Additionally, several industry leaders have formed strategic partnerships and pursued acquisitions to enhance their expertise in viral vector production and gene therapy manufacturing. Regulatory agencies are also working to streamline approval processes, facilitating the faster commercialization of gene-based treatments. With ongoing progress in genetic medicine, the market is expected to continue evolving with sustained innovation and expansion in the coming years.
Viral Vectors & Plasmid DNA Manufacturing Market Companies
- Novasep
- Aldevron
- MerckWaismanBiomanufacturing
- Creative Biogene
- The Cell and Gene Therapy Catapult
- Cobra Biologics
- uniQure N.V.
Segment Covered In The Report
By Vector Type
- Adenovirus
- Plasmid DNA
- Lentivirus
- Retrovirus
- AAV
- Others
By Application
- Gene Therapy
- Antisense &RNAi
- Cell Therapy
- Vaccinology
By Workflow
- Upstream Processing
- Vector Recovery/Harvesting
- Vector Amplification & Expansion
- Downstream Processing
- Fill-finish
- Purification
By End-User
- Biopharmaceutical and Pharmaceutical Companies
- Research Institutes
By Disease
- Genetic Disorders
- Cancer
- Infectious Diseases
- Others
By Geography
- North America
- U.S.
- Canada
- Europe
- U.K.
- Germany
- France
- Asia Pacific
- China
- India
- Japan
- South Korea
- Rest of the World
Ready for more? Dive into the full experience on our website!
https://www.precedenceresearch.com/