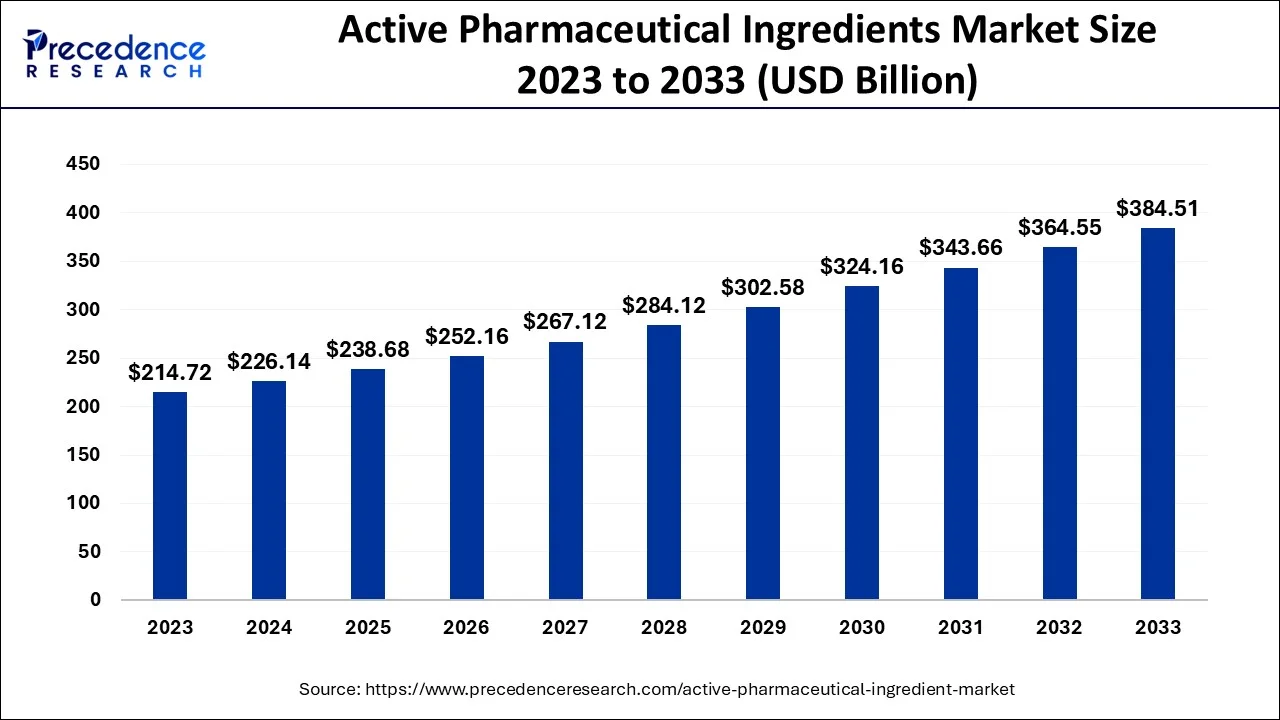
The global active pharmaceutical ingredients market was valued at USD 214.72 billion in 2023 and is projected to reach USD 384.51 billion by 2033, growing at a CAGR of 6.08%.
Active Pharmaceutical Ingredients Market Key Takeaways
- North America led the market with a revenue share of 38.31% in 2023.
- Asia Pacific is experiencing the highest CAGR of 6.37% from 2024 to 2033.
- The captive API segment dominated by manufacturer type, holding 57.26% revenue share in 2023.
- By type, innovative APIs accounted for 65.30% of total revenue in 2023.
- Cardiovascular diseases held a 20.99% revenue share in 2023, while oncology is growing at a 6.1% CAGR.
- Synthetic APIs captured 71.73% of the market share in 2023, while the biotech segment is expanding at a 5.9% CAGR.
Overview
The active pharmaceutical ingredients market is a crucial segment of the pharmaceutical industry, responsible for the production of the biologically active substances used in medications. APIs are the key components in drugs that provide therapeutic effects for various diseases, including cardiovascular disorders, neurological conditions, and infectious diseases. The market is growing due to rising demand for generic drugs, increasing chronic disease prevalence, and technological advancements in drug manufacturing. The expansion of biopharmaceuticals and the shift toward specialty medicines are further driving the evolution of the API industry. Additionally, regulatory agencies are implementing stringent quality and safety standards, influencing API manufacturing practices globally.
Drivers
The active pharmaceutical ingredients market is driven by several factors, including the growing global burden of chronic diseases such as diabetes, cancer, and cardiovascular disorders, which has increased the demand for effective medications. The rising geriatric population also contributes to market growth as older individuals require continuous medical treatment. Additionally, the increasing adoption of biosimilars and biologics has fueled the demand for high-quality APIs. Pharmaceutical companies are investing in research and development to create innovative drug formulations, further boosting the market. Cost efficiency and outsourcing API production to countries with lower manufacturing costs have also played a significant role in market expansion.
Opportunities
Opportunities in the active pharmaceutical ingredients market lie in the growing trend of personalized medicine and targeted drug therapies, which require specialized APIs. The shift toward biologics and peptides presents a lucrative growth area for API manufacturers. Advances in green chemistry and sustainable production techniques are also opening new possibilities for environmentally friendly API manufacturing. Additionally, the increasing demand for contract manufacturing organizations (CMOs) and contract development and manufacturing organizations (CDMOs) offers opportunities for companies to expand their production capabilities. The rise of digital technology and artificial intelligence in drug development is expected to enhance the efficiency and speed of API production.
Challenges
Despite its strong growth potential, the API market faces challenges such as stringent regulatory requirements, which can lead to delays in product approvals and increased compliance costs. The rising cost of raw materials and supply chain disruptions, particularly in key API-producing countries, pose risks to market stability. Intellectual property concerns and patent expirations also impact market dynamics, leading to increased competition among generic and branded drug manufacturers. Additionally, maintaining high-quality standards in API production is a constant challenge, as any deviation can affect drug efficacy and safety.
Regional Insights
North America dominates the API market due to strong pharmaceutical industry presence, high healthcare spending, and advanced manufacturing capabilities. The United States is a key player, with numerous pharmaceutical companies investing in API research and development. Europe follows closely, driven by strict regulatory frameworks and significant investment in biotechnology and biologics. The Asia-Pacific region is experiencing rapid growth, particularly in China and India, which are major hubs for API production due to cost-effective manufacturing and a well-established pharmaceutical supply chain. Latin America and the Middle East & Africa are also seeing steady growth, supported by improving healthcare infrastructure and increasing pharmaceutical investments.
Recent News
Recent developments in the API market include strategic mergers and acquisitions by major pharmaceutical companies to strengthen their API production capabilities. Several companies have announced investments in new API manufacturing facilities to meet growing global demand. Regulatory agencies are also updating guidelines to ensure API quality and safety compliance. Advances in biopharmaceutical APIs, particularly in monoclonal antibodies and gene therapy, are shaping the future of the market. Additionally, supply chain diversification strategies are being implemented to reduce dependency on a single region for API sourcing, ensuring a more resilient pharmaceutical supply network.
Active Pharmaceutical Ingredient (API) Market Companies
- Albemarle Corporation
- AurobindoPharma
- Reddy’s Laboratories Ltd.
- AbbVieInc
- Teva Pharmaceutical Industries Ltd
- Mylan N.V.
- CiplaInc
Segments Covered in the Report
By Type of Synthesis
- Biotech
- Monoclonal Antibodies
- Recombinant Proteins
- Vaccines
- Synthetic
By Type of Manufacturer
- Captive APIs
- Merchant APIs
- Generic APIs
- Innovative APIs
By Type
- Generic APIs
- Innovative APIs
By Application
- Cardiovascular Diseases
- Oncology
- CNS & Neurological Disorders
- Orthopedic Disorders
- Endocrinology
- Pulmonology
- Gastrointestinal Disorders
- Nephrology
- Ophthalmology
- Others
By Regional Outlook
- North America
- U.S.
- Canada
- Europe
- U.K.
- Germany
- France
- Asia Pacific
- China
- India
- Japan
- South Korea
- Middle East & Africa
- Latin America