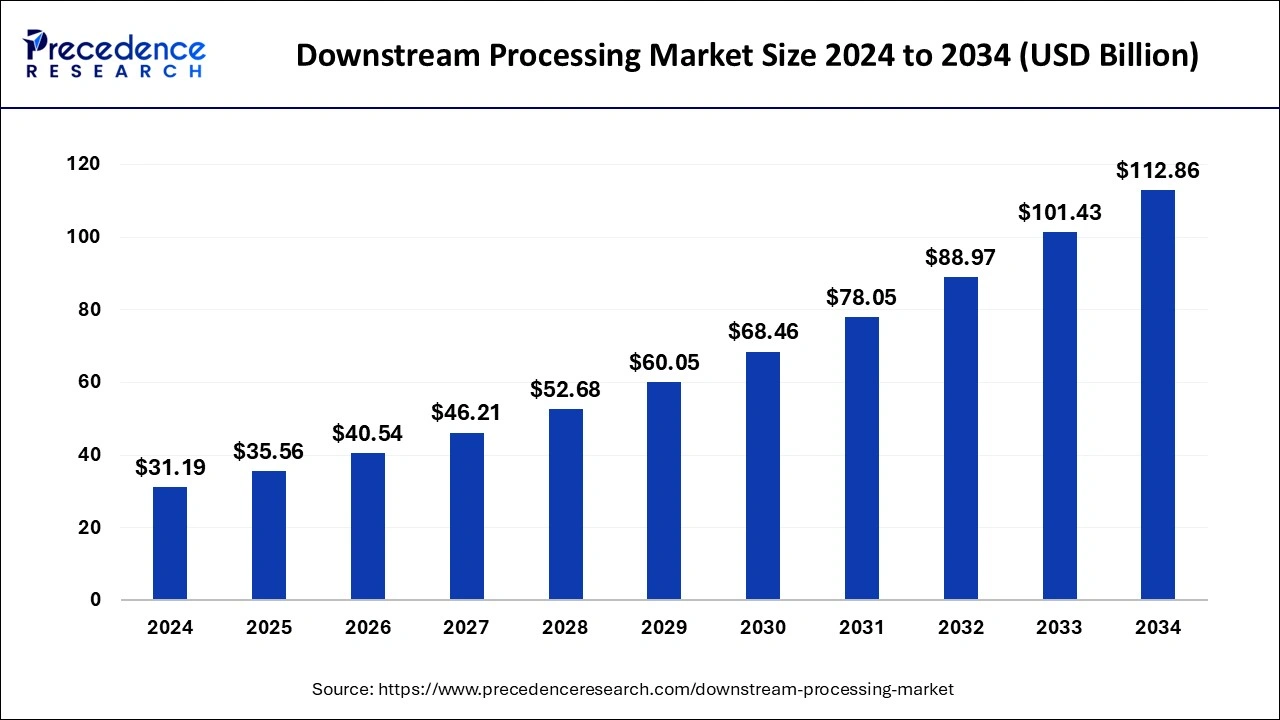
The global downstream processing market is projected to grow from USD 31.19 billion in 2024 to USD 112.86 billion by 2034, with a CAGR of 13.72%.
Downstream Processing Market Key Takeaways
- North America dominated the global market with a major market share of 36.14% in 2024.
- Asia-Pacific is expected to expand at the fastest CAGR between 2025 and 2034.
- By Application, the antibiotic production segment generated revenue share of 34% in 2024.
- By Technique, the monoclonal antibody medication segment contributed more than 42% of revenue share in 2024.
- By Product, the thalassemia segment has held the biggest revenue share of 43% in 2024.
Market Overview
The downstream processing market is witnessing rapid growth due to the increasing demand for biopharmaceuticals, vaccines, and biosimilars. Downstream processing refers to the recovery, purification, and polishing of biological products derived from cell culture or fermentation processes. This process is critical in the biotechnology and pharmaceutical industries as it ensures that the final product meets the required quality, safety, and efficacy standards.
Downstream processing consists of several steps, including cell disruption, solid-liquid separation, purification, and polishing. These steps are essential for removing impurities and ensuring that the final product is free from contaminants. The growing focus on personalized medicine, the increasing prevalence of chronic diseases, and the rise in research and development activities in biopharmaceuticals have fueled the demand for advanced downstream processing technologies.
The market is also benefiting from technological advancements in chromatography, filtration systems, and membrane technologies that enable faster and more efficient downstream processing. Furthermore, the adoption of single-use technologies and continuous processing techniques is improving operational efficiency and reducing production costs, driving the growth of the downstream processing market.
Drivers
-
Rising Demand for Biopharmaceuticals and Biosimilars
The increasing demand for biopharmaceuticals, including monoclonal antibodies, recombinant proteins, and vaccines, is a major driver for the downstream processing market. As biopharmaceuticals require complex purification processes, downstream processing technologies play a crucial role in ensuring product quality and safety. -
Growing Investment in R&D for Biologics and Gene Therapy
Pharmaceutical and biotechnology companies are investing heavily in research and development (R&D) to develop innovative biologics, gene therapies, and cell therapies. These advanced therapies require sophisticated downstream processing solutions to meet stringent regulatory standards and ensure high yield and purity. -
Increased Adoption of Single-Use Technologies
Single-use technologies are gaining popularity in downstream processing due to their ability to reduce cross-contamination risks, minimize downtime, and lower operational costs. The adoption of disposable systems is driving the market’s growth by enhancing flexibility and efficiency in biopharmaceutical manufacturing. -
Regulatory Emphasis on Quality and Compliance
Stringent regulatory guidelines set by regulatory authorities such as the FDA and EMA are driving the adoption of high-quality downstream processing solutions. Manufacturers are focusing on complying with Good Manufacturing Practices (GMP) and ensuring product consistency, thereby increasing demand for advanced downstream processing technologies.
Opportunities
-
Expansion of Biosimilar Production
The expiration of patents for several biologic drugs has led to an increase in the production of biosimilars. As biosimilars require similar downstream processing techniques to ensure quality and efficacy, this trend presents a significant opportunity for market growth. -
Rising Demand for Personalized Medicine and Precision Therapies
The growing focus on personalized medicine and precision therapies is creating opportunities for downstream processing technologies. These therapies require highly specific purification and polishing techniques to meet the unique needs of individual patients. -
Emergence of Continuous Bioprocessing Technologies
The shift from traditional batch processing to continuous bioprocessing is revolutionizing downstream processing by improving efficiency, reducing processing time, and enhancing product consistency. Continuous processing offers a more streamlined and cost-effective approach, opening new growth avenues for market players.
Challenges
-
High Operational Costs and Capital Investment
The high cost associated with downstream processing equipment, consumables, and maintenance poses a challenge for small and medium-sized enterprises (SMEs). Capital-intensive infrastructure and operational costs can limit the adoption of advanced downstream processing technologies. -
Complexity in Purification of Biologics
The purification of biologics is a complex and time-consuming process that requires advanced technologies and expertise. The complexity of downstream processing increases the risk of product loss, making it a challenge for manufacturers to achieve high yields. -
Stringent Regulatory Requirements
Compliance with stringent regulatory guidelines and quality standards adds complexity to downstream processing. Meeting regulatory requirements for product safety, efficacy, and consistency requires significant investment in quality control and validation processes.
Regional Insights
-
North America
North America dominates the downstream processing market due to the presence of a well-established biopharmaceutical industry and advanced research infrastructure. The United States, in particular, leads the market with high investments in biologics and gene therapy research. Regulatory support and government funding for biopharmaceutical innovations further boost market growth in the region. -
Europe
Europe holds a significant share of the downstream processing market, driven by increasing demand for biosimilars and the growing focus on personalized medicine. Countries such as Germany, the UK, and France are leading contributors, with strong investments in biopharmaceutical manufacturing and research. -
Asia Pacific
The Asia Pacific region is witnessing rapid growth in the downstream processing market due to the rising demand for biopharmaceuticals, increasing government support for biotechnology research, and expanding contract manufacturing services. Countries like China, India, and South Korea are emerging as key hubs for biologics and biosimilar production. -
Latin America and Middle East & Africa
Latin America and the Middle East & Africa regions are gradually gaining traction in the downstream processing market due to increasing investments in healthcare infrastructure and biotechnology research. Growing awareness about the benefits of biologics and improving regulatory frameworks are contributing to market growth.
Recent News
-
Expansion of Manufacturing Facilities for Biologics
Leading biopharmaceutical companies are expanding their manufacturing facilities to meet the rising demand for biologics and biosimilars. This expansion is driving the adoption of advanced downstream processing technologies. -
Introduction of Advanced Chromatography and Filtration Systems
Several companies have introduced innovative chromatography and filtration systems to enhance the efficiency and scalability of downstream processing, catering to the growing demand for high-purity biologics.
Downstream Processing Market Companies
- BoehringerIngelheim
- Danaher Corporation
- Sartorius Stedium Biotech S.A
- GE Healthcare
- Merck Millipore
- Eppendorf AG
- 3M Company
- Thermo Fisher Scientific Inc
- Finesse Solutions Inc
- Lonza Group
Segments Covered in the Report
By Product
- Centrifuges
- Dryers
- Chromatography Systems
- Filters
- Evaporators
- Others
By Application
- Antibodies Production
- Antibiotic Production
- Hormone Production
- Enzyme Production
- Vaccine Production
By Technique
- Purification by Chromatography
- Solid-liquid Separation
- Centrifugation
- Filtration
- Cell Disruption
- Concentration
- Formulation
By Geography
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East & Africa