Real-World Data (RWD) Market Key Takeaways
- North America led the market by holding 43% of market share in 2024.
- Asia Pacific is projected to grow at a solid CAGR of 11% in the coming years.
- By component, the services segment held a dominant presence in the market in 2024.
- By component, the datasets segment is expected to grow at the fastest rate during the forecast period of 2024 to 2034.
- By application, the drug development and approvals segment accounted for a considerable share of the market in 2024.
- By application, the post-market surveillance segment is anticipated to grow with the highest CAGR in the market during the studied years.
- By end-user, in 2024, the pharmaceutical and medical device companies segment led the global market.
- By end-user, the healthcare providers segment is projected to expand rapidly in the coming years.
Real World Data (RWD) Market Overview
The real world data (RWD) market is rapidly expanding due to the growing importance of data-driven decision-making in healthcare and clinical research. Real world data refers to information collected from various sources outside traditional clinical trials, including electronic health records (EHRs), claims data, patient registries, wearable devices, and social media. This data provides valuable insights into the effectiveness, safety, and outcomes of medical treatments in real-world settings, allowing healthcare providers, pharmaceutical companies, and regulatory bodies to make informed decisions.
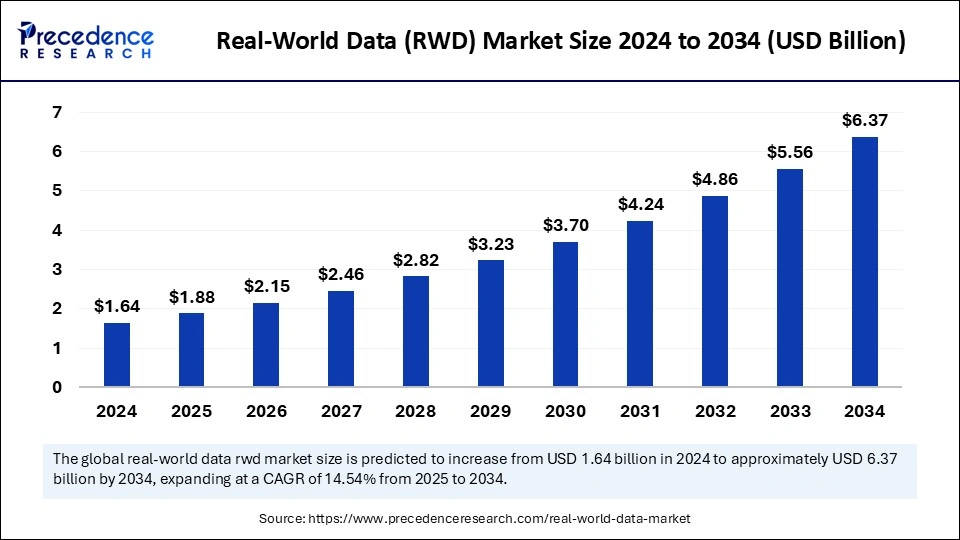
The global real world data market size is expected to witness significant growth between 2024 and 2034, driven by the increasing demand for evidence-based healthcare solutions, the rising prevalence of chronic diseases, and advancements in data analytics and artificial intelligence (AI). Real world data plays a crucial role in improving patient outcomes, optimizing treatment protocols, and supporting regulatory approvals by providing a comprehensive understanding of treatment effectiveness beyond controlled clinical environments. As healthcare organizations increasingly recognize the value of RWD in enhancing patient care and reducing healthcare costs, the adoption of real world data solutions is expected to accelerate.
Drivers of the Real World Data (RWD) Market
-
Growing Demand for Evidence-Based Medicine:
The shift towards evidence-based healthcare practices is driving the adoption of real world data solutions. RWD provides insights into how treatments perform in real-world scenarios, helping healthcare providers optimize patient care and improve treatment outcomes. -
Increasing Adoption of Electronic Health Records (EHRs):
The widespread implementation of EHR systems across healthcare facilities is generating vast amounts of patient data. This data is being leveraged to derive real world insights, enabling healthcare providers to make more informed clinical decisions. -
Rising Prevalence of Chronic Diseases:
The growing burden of chronic diseases such as diabetes, cardiovascular diseases, and cancer is increasing the need for real world data to evaluate long-term treatment effectiveness and patient outcomes. RWD helps identify trends, assess treatment efficacy, and develop personalized treatment plans. -
Regulatory Support for Real World Evidence (RWE):
Regulatory agencies such as the US FDA and the European Medicines Agency (EMA) are increasingly recognizing the importance of real world evidence derived from RWD in supporting drug approvals and post-market surveillance. This regulatory support is driving the adoption of RWD solutions by pharmaceutical companies and healthcare organizations.
Opportunities in the Real World Data (RWD) Market
-
Integration of Artificial Intelligence and Machine Learning:
The integration of AI and machine learning with real world data platforms is enhancing the ability to analyze large datasets and generate actionable insights. These technologies can identify patterns, predict outcomes, and improve the accuracy of treatment recommendations. -
Growing Use of Wearable Devices and IoT Sensors:
The proliferation of wearable devices and IoT sensors is generating a wealth of real world data related to patient health and behavior. This data can be leveraged to monitor chronic conditions, detect anomalies, and personalize treatment plans, creating new opportunities for market growth. -
Expansion of RWD Applications in Clinical Trials:
Real world data is increasingly being used to complement traditional clinical trials by providing real-time insights into patient outcomes and treatment responses. This trend is opening new avenues for integrating RWD into clinical research and drug development processes.
Challenges Facing
-
Data Privacy and Security Concerns:
The collection and use of real world data raise concerns about patient privacy and data security. Ensuring compliance with data protection regulations such as HIPAA and GDPR is a significant challenge for organizations leveraging RWD. -
Data Quality and Standardization Issues:
Real world data is collected from diverse sources, often leading to inconsistencies and variations in data quality. Standardizing and harmonizing data from multiple sources to ensure accuracy and reliability remains a major challenge. -
Limited Awareness and Adoption of RWD Solutions:
Despite the growing recognition of the value of real world data, many healthcare organizations and research institutions are still in the early stages of adopting RWD solutions. Increasing awareness and promoting the benefits of RWD is essential for driving widespread adoption.
Regional Analysis
North America:
North America dominates the real world data market, with the United States being the largest contributor. The region’s strong healthcare infrastructure, widespread adoption of EHR systems, and favorable regulatory environment are driving the growth of the RWD market. The US FDA’s emphasis on real world evidence in regulatory decision-making is further fueling market expansion.
Europe:
Europe is witnessing significant growth in the real world data market due to increasing government initiatives to promote data-driven healthcare practices. Countries such as the UK, Germany, and France are at the forefront of adopting RWD solutions to improve patient care and optimize treatment outcomes. The European Medicines Agency’s guidelines on using real world evidence are encouraging pharmaceutical companies to leverage RWD for regulatory submissions.
Asia Pacific:
Asia Pacific is expected to experience the fastest growth in the real world data market, driven by the rapid adoption of digital health technologies and increasing investments in healthcare infrastructure. Countries such as China, India, and Japan are embracing real world data solutions to address the challenges of managing large patient populations and improving healthcare delivery.
Middle East and Africa:
The Middle East and Africa region is gradually adopting real world data solutions to enhance healthcare outcomes and optimize resource utilization. Governments in the region are investing in healthcare digitization initiatives to improve data collection and analysis capabilities.
Recent News
-
In February 2025, a leading data analytics company launched an AI-powered RWD platform designed to enhance clinical research and improve patient outcomes.
-
In March 2025, a major pharmaceutical company partnered with a real world data provider to leverage RWD in post-market surveillance and regulatory submissions.
-
In January 2025, a government healthcare agency announced a new initiative to promote the use of real world data in healthcare decision-making and policy development.
Real-World Data (RWD) Market Companies
- Cerner Corporation
- Evidera, Inc.
- Flatiron Health, Inc.
- IBM Corporation
- IQVIA Holdings Inc.
- Optum, Inc. (a subsidiary of UnitedHealth Group)
- Palantir Technologies Inc.
- SAS Institute Inc.
- Syneos Health Inc.
- Tempus Labs Inc.
Segments Covered in the Report
By Component
- Services
- Datasets
By Application
- Drug Development & Approvals
- Market Access & Reimbursement/Coverage Decisions
- Post-market Surveillance
- Clinical Research
- Other
By End User
- Pharmaceutical & Medical Device Companies
- Healthcare Payers
- Healthcare Providers
- Government Agencies
- Others
By Geography
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa