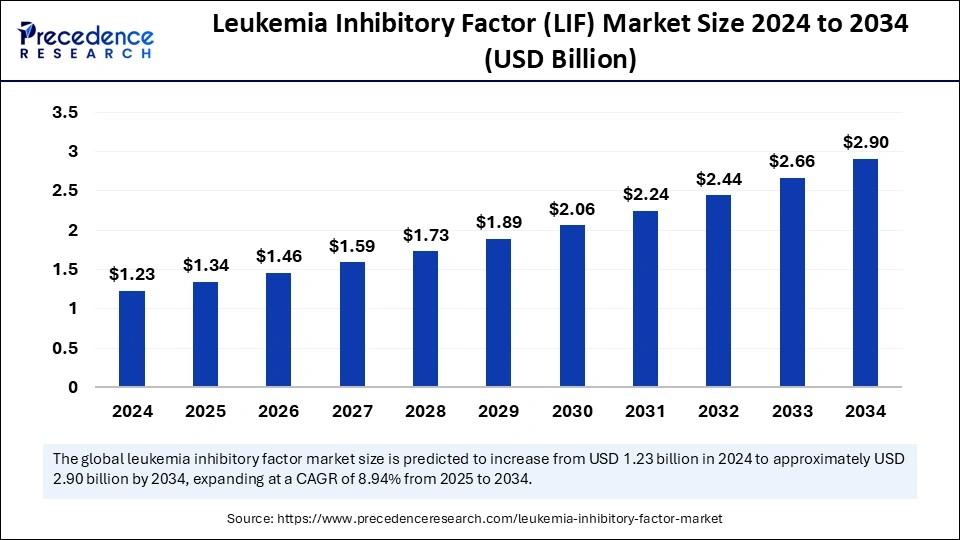
Leukemia Inhibitory Factor (LIF) market is projected to grow from USD 1.23 billion in 2024 to USD 2.90 billion by 2034, at a CAGR of 8.94%
Leukemia Inhibitory Factor (LIF) Market Key Takeaways
-
North America led the leukemia inhibitory factor (LIF) market with a 41% share in 2024.
-
Asia Pacific is projected to experience rapid growth throughout the forecast period.
-
Europe is expanding at a notable CAGR, showing strong market potential.
-
The recombinant LIF segment dominated the market in 2024.
-
The LIF antibodies segment is expected to witness significant growth in the coming years.
-
The cancer treatment segment held the highest market share in 2024.
-
The stem cell research segment is projected to grow at the fastest rate over the forecast period.
-
Hospitals accounted for the largest market share in 2024.
-
Research institutes are anticipated to expand rapidly in the coming years.
Leukemia Inhibitory Factor (LIF) Market Overview
The Leukemia Inhibitory Factor (LIF) Market is gaining traction due to its crucial role in cell differentiation, stem cell research, and regenerative medicine. LIF, a cytokine belonging to the interleukin-6 family, is widely studied for its ability to maintain embryonic stem cell pluripotency and its potential applications in treating neurological disorders, cancer, and autoimmune diseases.
Growing research in regenerative medicine and advancements in cell-based therapies have increased the demand for LIF-based treatments. Additionally, its involvement in neuroprotection and fertility treatments is expanding its market potential, as researchers explore new therapeutic applications beyond oncology.
Leukemia Inhibitory Factor (LIF) Market Drivers
The rising prevalence of chronic diseases, including neurodegenerative disorders, autoimmune conditions, and cancer, is a significant driver of the Leukemia Inhibitory Factor market. Increasing investment in stem cell research and regenerative medicine is also fueling demand, as LIF plays a crucial role in maintaining stem cell viability. Government funding and private sector initiatives supporting advanced biotechnological research have further accelerated market growth.
Additionally, the growing focus on personalized medicine and targeted therapies has boosted interest in LIF’s role in cell signaling and tissue regeneration. The expansion of fertility treatments, particularly in vitro fertilization (IVF), where LIF is essential for embryo implantation, is another key factor contributing to market growth.
Leukemia Inhibitory Factor (LIF) Market Opportunities
The market presents significant opportunities in the field of regenerative medicine, where LIF’s ability to regulate cell differentiation and tissue repair opens avenues for novel treatments. Research into neurodegenerative diseases such as Alzheimer’s and Parkinson’s is uncovering potential therapeutic applications, offering a lucrative market segment.
Additionally, the development of recombinant LIF products for cell culture and biopharmaceutical research creates opportunities for manufacturers. The integration of artificial intelligence and bioinformatics in drug discovery is further enabling the identification of new roles for LIF in disease management. Collaborations between biotechnology firms and academic institutions are also paving the way for innovative applications, particularly in cancer immunotherapy.
Leukemia Inhibitory Factor (LIF) Market Challenges
Despite its promising potential, the Leukemia Inhibitory Factor market faces several challenges. High costs associated with LIF-based therapies and stem cell treatments can limit accessibility, particularly in developing regions. Regulatory hurdles for new biopharmaceutical applications add complexity to market entry, as stringent approval processes require extensive clinical trials and safety assessments.
Additionally, ethical concerns surrounding stem cell research may impact public perception and funding. Technical challenges in large-scale production and maintaining LIF stability also pose obstacles for manufacturers, as ensuring product consistency remains a critical issue in biopharmaceutical development.
Leukemia Inhibitory Factor (LIF) Market Regional Insights
North America leads the LIF market due to robust investment in biotechnology, well-established research institutions, and increasing demand for regenerative medicine applications. The United States, in particular, has a strong presence of biotechnology firms developing LIF-based products for stem cell research and cancer treatment. Europe follows closely, with growing government support for stem cell therapy and neurodegenerative disease research.
The Asia-Pacific region is witnessing rapid growth, driven by increasing investments in biomedical research, rising healthcare expenditures, and expanding fertility treatment centers. China and Japan are key players in the region, leveraging their advancements in biopharmaceutical research. Latin America and the Middle East & Africa have emerging markets, but limited research infrastructure and regulatory challenges may slow down market expansion.
Leukemia Inhibitory Factor (LIF) Market Recent Developments
The LIF market has seen several recent advancements, including increased research on its role in neurodegenerative diseases and cancer immunotherapy. Biopharmaceutical companies are developing recombinant LIF formulations for cell culture and therapeutic applications. Collaborations between research institutions and biotechnology firms have led to promising clinical trials exploring LIF’s regenerative potential.
Regulatory agencies are also reviewing novel LIF-based treatments, paving the way for new market approvals. Advances in gene editing and stem cell technology have further enhanced the understanding of LIF’s role in tissue regeneration and personalized medicine.
- In October 2024, Novartis announced the approval from the U.S. Food and Drug Administration (FDA) for the Scemblix® (asciminib) for adult patients who are newly diagnosed with Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase (Ph+ CML-CP).
- In February 2024, Johnson & Johnson in collaboration with Pharmacyclics LLC announced the approval from the U.S. Food and Drug Administration (FDA) for expanding IMBRUVICA® (ibrutinib) with an oral suspension formulation for adult patients going through the treatment of chronic lymphocytic leukemia (CLL), and other related diseased conditions after failure of one or more lines of systemic therapy.
Leukemia Inhibitory Factor (LIF) Market Companies
- Bristol Myers Squibb
- InVitria
- Johnson & Johnson Private Limited
- Gilead Sciences Inc.
- Sanofi S.A.
- Novartis AG
- Roche
- Amgen Inc.
- Merck KGaA
Segments Covered in the Report
By Product Type
- Recombinant LIF
- LIF Antibodies
- LIF Receptor Agonists/Antagonists
By Application
- Cancer Treatment
- Stem Cell Research
- Neurological Disorders
- Fertility Treatment
- Others
By End-User
- Hospitals
- Research Institutes
- Biotechnology Companies
- Others
By Geography
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East & Africa
Ready for more? Dive into the full experience on our website!