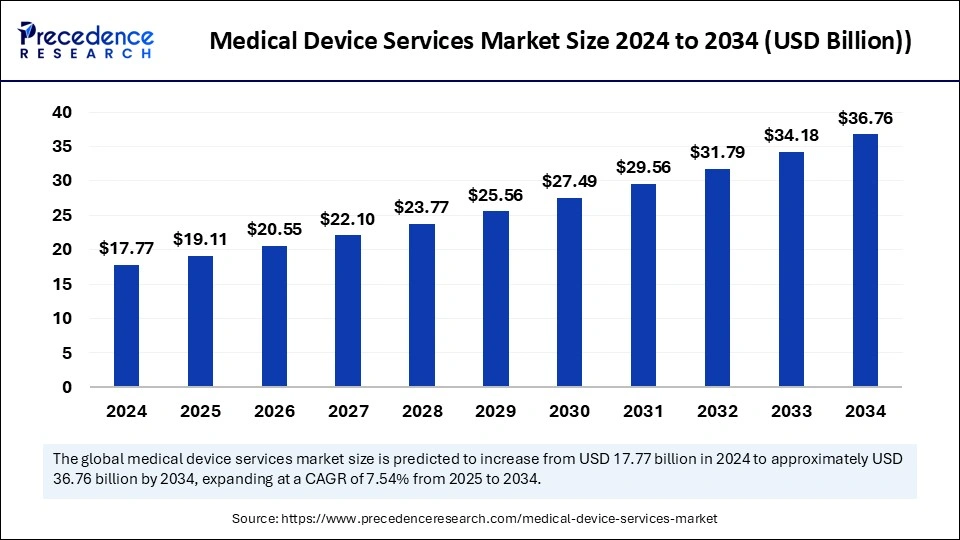
Medical Device Services Market Key Takeaways
-
In 2024, North America dominated the medical device services market; Asia Pacific is expected to lead in growth.
-
Europe shows stable, sustained growth throughout the forecast period.
-
Manufacturing services were the largest segment by service type, while product design & development is emerging strongly.
-
Surgical devices led the market by device type in 2024; patient monitoring devices to rise rapidly.
-
Medical device manufacturers held the top spot by end-user; healthcare facilities are gaining traction.
Medical Device Services Market Overview
The Medical Device Testing Services sector plays a crucial role in ensuring that medical devices meet necessary safety, efficacy, and regulatory standards before reaching the market. This segment encompasses a range of services, including performance testing, biocompatibility assessments, and sterilization validation.
The medical device services market evidenced rapid growth owing to technological developments, including telehealth, and the increasing significance of wearable health devices and patient-centered care. The growth number of patients with chronic illness including cancer, diabetes, and cardiovascular diseases.
As healthcare progress demands better, sophisticated medical devices, the medical sector faces an expanding necessity for more innovative equipment. The rise in patient numbers creates substantial demand for diagnostic and surgical procedures, stimulating market requirements for medical devices and supporting services.
Medical Device Services Market Drivers
The increasing complexity of medical devices, incorporating advanced technologies like AI and biotechnology, necessitates rigorous testing protocols to ensure patient safety and device reliability. Additionally, stringent regulatory requirements imposed by agencies such as the U.S. Food and Drug Administration (FDA) and the European Union’s Medical Device Regulation (MDR) drive the demand for comprehensive testing services.
Opportunities
The adoption of connected and digital health technologies, including IoT-enabled devices and telemedicine solutions, presents significant opportunities for testing service providers. As these technologies become more prevalent, there is a growing need for services that address cybersecurity, interoperability, and data privacy concerns, ensuring that devices function seamlessly within the broader healthcare ecosystem.
Challenges
The high costs associated with meeting rigorous regulatory guidelines pose a considerable challenge for manufacturers and service providers alike. Compliance requires significant investment in testing, documentation, and certification processes. Additionally, the rapidly evolving regulatory landscape necessitates continuous monitoring and adaptation to maintain compliance.
Regional Insights
Asia-Pacific held approximately 41% of the market share in 2023, reflecting the region’s growing prominence in medical device manufacturing and testing services. Factors contributing to this dominance include favorable government policies, a skilled workforce, and cost-effective service offerings.
Recent Developments
The integration of advanced technologies, such as AI and machine learning, into testing processes is enhancing the efficiency and accuracy of assessments. Additionally, there is an increasing emphasis on developing standardized testing protocols for emerging device categories, including wearable and connected medical devices, to address unique challenges related to connectivity and data security.
Medical Device Services Market Top Companies
- Wipro
- IQVIA
- TATA Consultancy Services Limited
- UL LLC
- SGS
- Cognizant
- Dragerwerk AG and Co. KGaA
- Eurofins Scientific
- L&T Technology Services Limited
- Infosys Limited
- DEKRA
- IBM
- Charles River Laboratories
- Intertek Group plc
- Cardinal Health
- Brighter Health Network, LLC
- Others
Segments Covered in the Market
By Service Type
- Regulatory and quality compliance
- Product design and development
- Product testing and certification
- Manufacturing services
- Maintenance and calibration
- Post-market surveillance
- Others (it and software solutions, supply chain management)
By Device Type
- Diagnostic devices
- Therapeutic devices
- Surgical devices
- Patient monitoring devices
- Others
By End-User
- Medical device manufacturers
- Healthcare facilities
- Hospitals
- Clinics
- Diagnostic laboratories
- Others
- Academic institutions and research organizations
- Others
By Region
- North America
- Asia Pacific
- Europe
- Latin America
- Middle East and Africa
Ready for more? Dive into the full experience on our website!