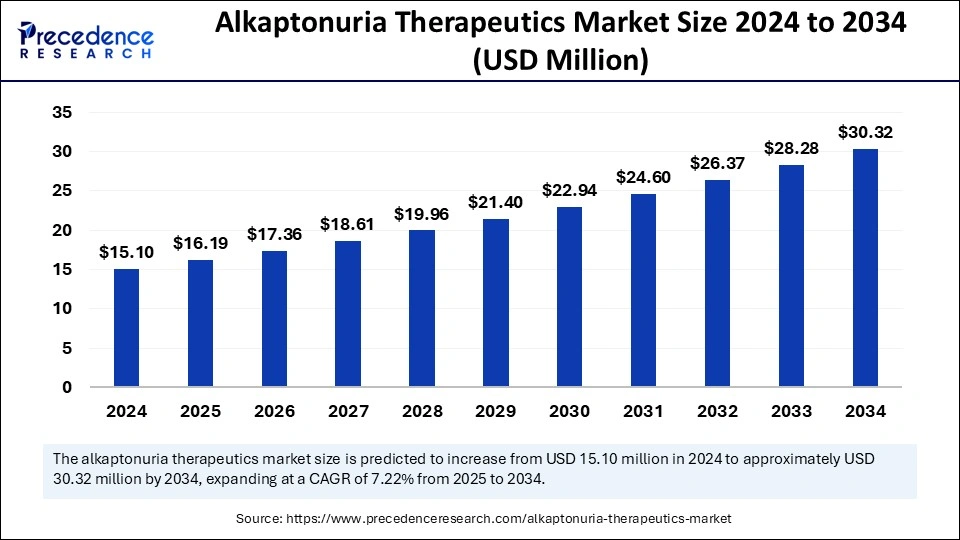
Alkaptonuria Therapeutics Market Key Takeaways
- North America dominated the alkaptonuria therapeutics market in 2024.
- Asia Pacific is projected to grow at the fastest CAGR in the coming years.
- By drug type, the nitisinone (p-hydroxyphenyl-pyruvate dioxygenase inhibitor) segment held the largest market in 2024.
- By drug type, the dietary supplements segment is expected to grow at a solid CAGR during the forecast period of 2025 to 2034.
- By route of administration, the oral segment accounted for a considerable share of it in 2024.
- By rote of administration, the parenteral segment is expected to grow at a highest CAGR in the studied years.
- By patient age group, the adult segment led the global market in 2024.
- By patient age group, the pediatric segment is projected to expand rapidly in the coming years.
- By distribution channel, the hospital pharmacies segment dominated the global market in 2024.
- By distribution channel, the online pharmacies segment is projected to grow at the fastest CAGR in the future years.
Alkaptonuria Therapeutics Market Overview
The Alkaptonuria therapeutics market is rooted in the urgent need for effective treatments for a debilitating and lifelong condition. Alkaptonuria, though rare, leads to severe complications including ochronosis and early-onset osteoarthritis. For many patients, available treatments have historically focused on symptom relief rather than disease modification. The introduction of targeted therapies is slowly changing this narrative, making the market one of hope and ongoing evolution.
Drivers
Patient advocacy and increased disease visibility have been powerful drivers behind recent progress. Access to genetic screening and support from global rare disease networks are pushing for earlier diagnoses and better disease management. Moreover, the pharmaceutical industry’s growing social commitment to address unmet medical needs in rare diseases is adding momentum to therapeutic advancements.
Opportunities
There’s a real chance to change lives with advancements in precision medicine. The integration of AI in rare disease research could significantly shorten the drug discovery timeline. Enhanced patient registries and global data-sharing initiatives offer new ways to conduct decentralized clinical trials, increasing inclusivity and representation in research efforts. Additionally, nutraceuticals and supportive therapies may provide adjunctive benefits in improving patient quality of life.
Challenges
Patients often face long diagnostic journeys due to the non-specific symptoms of Alkaptonuria. The emotional and financial burden is compounded by limited treatment options and inconsistent access to care across different regions. Pharmaceutical developers also face ethical and logistical issues when designing trials for such small populations, which can delay innovation.
Regional Insights
Western countries with strong rare disease frameworks, such as the United States, the United Kingdom, and France, lead in both patient support and treatment availability. In contrast, countries in Asia, Africa, and Latin America often lack diagnostic infrastructure and national rare disease policies, resulting in significant disparities in care. However, increasing global awareness campaigns are beginning to bridge this gap.
Recent Developments
Nitisinone remains the cornerstone therapy for Alkaptonuria and has been approved for use in certain regions following positive outcomes in clinical studies. Several biotech companies have launched early-stage trials for next-gen therapies. Furthermore, interdisciplinary care models integrating orthopedic, renal, and cardiovascular support are emerging, reflecting a more holistic approach to managing the disease.
Alkaptonuria Therapeutics Market Companies
- Bristol Myers Squibb
- Cipla
- Dr. Reddy’s Laboratories Ltd.
- Eton Pharmaceuticals
- GSK plc
- Johnson & Johnson Services, Inc.
- Mallinckrodt Pharmaceuticals
- Pfizer Inc.
- Sanofi
- Sun Pharmaceutical Industries Ltd.
- Swedish Orphan Biovitrum AB
- Teva Pharmaceutical Industries Ltd.
Segments Covered in the Report
By Drug Type
- Dietary Supplements
- Nitisinone (p-hydroxyphenyl-pyruvate dioxygenase inhibitor)
- Pain Relief Medication
By Route of Administration
- Oral
- Parenteral
- Topical
By Patient Age Group
- Adult
- Geriatric
- Pediatric
By Distribution Channel
- Hospital Pharmacies
- Online Pharmacies
- Retail Pharmacies
By Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa
Ready for more? Dive into the full experience on our website!