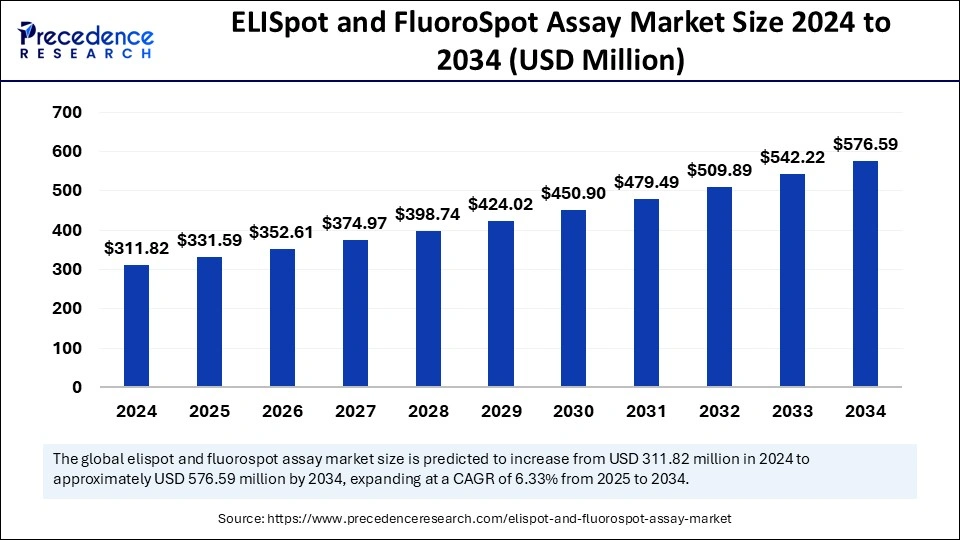
ELISpot and FluoroSpot assay market is projected to grow from USD 311.82 million in 2024 to USD 576.59 million by 2034, at a CAGR of 6.33%.
ELISpot and FluoroSpot Assay Market Key Takeaways
-
North America captured the largest market share of 35% in 2024, with Asia Pacific forecasted to grow at the highest CAGR of 7.24% throughout the forecast period.
-
Europe is expected to demonstrate notable growth in the coming years.
-
Assay kits dominated by product with a 50% market share in 2024, while analyzers are likely to grow at an impressive rate between 2025 and 2034.
-
Diagnostic applications contributed the largest share of 67% by application in 2024, while research applications are projected to expand at the fastest rate.
-
The hospital and clinical labs segment accounted for 47% of the market by end-use in 2024, while biopharmaceutical companies are poised to show rapid growth during the forecast timeframe.
ELISpot and FluoroSpot Assay Market Overview
The ELISpot and FluoroSpot Assay Market is witnessing significant growth, driven by the rising demand for advanced immunoassay techniques that provide accurate, sensitive, and high-throughput analysis of immune responses. Enzyme-linked immunospot (ELISpot) and FluoroSpot assays are widely used in immunological research, vaccine development, and clinical diagnostics. These assays enable the detection and quantification of cytokine-secreting cells at the single-cell level, making them essential for understanding immune responses in infectious diseases, cancer immunotherapy, and autoimmune disorders.
The increasing adoption of these assays for monitoring T-cell and B-cell responses in clinical research, coupled with technological advancements in assay platforms, is propelling market growth. The global ELISpot and FluoroSpot assay market is expected to grow steadily over the next decade, driven by the increasing need for immune monitoring in drug development, rising prevalence of infectious diseases, and expanding applications in cancer immunotherapy.
ELISpot and FluoroSpot Assay Market Drivers
-
Rising Prevalence of Infectious Diseases and Emerging Pandemics
The increasing incidence of infectious diseases, such as HIV, tuberculosis, and hepatitis, is driving the demand for accurate and reliable immune response monitoring. ELISpot and FluoroSpot assays play a crucial role in assessing immune responses, enabling timely diagnosis and monitoring of disease progression. -
Growing Focus on Cancer Immunotherapy and Vaccine Development
The expanding field of cancer immunotherapy, which leverages the immune system to combat cancer, is boosting the adoption of ELISpot and FluoroSpot assays. These assays are extensively used in evaluating immune responses to immunotherapeutic agents, monitoring vaccine efficacy, and assessing antigen-specific T-cell responses. -
Increasing Application in Autoimmune and Chronic Diseases
ELISpot and FluoroSpot assays are increasingly utilized in autoimmune disease research to analyze immune responses and identify biomarkers associated with disease progression. The growing prevalence of autoimmune conditions, such as rheumatoid arthritis and multiple sclerosis, is driving the adoption of these assays in clinical and translational research.
ELISpot and FluoroSpot Assay Market Opportunities
-
Expansion of Research in Infectious Disease Immunology
The ongoing research in infectious disease immunology, particularly in understanding the immune responses to viral and bacterial infections, presents significant opportunities for ELISpot and FluoroSpot assay manufacturers. Emerging pandemics and the need for rapid immune monitoring have further amplified these opportunities. -
Advancements in Multiplexing and High-Throughput Platforms
Technological advancements in multiplexing and high-throughput assay platforms are creating opportunities for improved immune response analysis. FluoroSpot assays, which enable the simultaneous detection of multiple cytokines, are gaining traction due to their ability to provide comprehensive immune profiling. -
Increasing Adoption in Biopharmaceutical and Clinical Trials
The growing reliance on ELISpot and FluoroSpot assays in biopharmaceutical research and clinical trials is driving market growth. These assays are critical for evaluating immune responses during vaccine and drug development, creating new opportunities for assay manufacturers and service providers.
ELISpot and FluoroSpot Assay Market Challenges
-
High Cost of Assay Kits and Reagents
The high cost associated with ELISpot and FluoroSpot assay kits, reagents, and equipment remains a barrier to widespread adoption, particularly in resource-limited settings. Organizations need to optimize cost-efficiency while maintaining assay quality to enhance accessibility. -
Complexity in Assay Standardization and Validation
Standardizing and validating ELISpot and FluoroSpot assays for different applications can be challenging, leading to variability in results. Ensuring assay reproducibility across laboratories remains a significant challenge for researchers and clinicians. -
Limited Expertise and Technical Knowledge
Successful execution of ELISpot and FluoroSpot assays requires specialized expertise and technical knowledge. The lack of skilled professionals proficient in assay optimization, data interpretation, and troubleshooting limits widespread adoption, particularly in developing regions.
ELISpot and FluoroSpot Assay Market Regional Insights
-
North America
North America dominates the ELISpot and FluoroSpot assay market, accounting for the largest market share due to the high prevalence of infectious diseases, robust research infrastructure, and significant investments in immunological research. The presence of leading biopharmaceutical companies and clinical research organizations further boosts market growth in the region. -
Europe
Europe is witnessing steady growth in the ELISpot and FluoroSpot assay market, driven by increasing government funding for immunology research and rising demand for advanced diagnostic techniques. Countries such as Germany, the UK, and France are at the forefront of assay adoption in clinical and academic research. -
Asia Pacific
The Asia Pacific region is poised to experience the fastest growth due to increasing investments in biotechnology and pharmaceutical research, rising healthcare expenditures, and growing awareness about immunotherapy. Countries like China, India, and Japan are emerging as key markets for ELISpot and FluoroSpot assays.
ELISpot and FluoroSpot Assay Market Recent Developments
-
Introduction of AI-Integrated Assay Platforms
Leading manufacturers are incorporating AI-based analytics and automation capabilities into ELISpot and FluoroSpot assay platforms to enhance assay performance and streamline data interpretation. -
Strategic Collaborations to Enhance Assay Portfolios
Industry players are entering strategic partnerships and collaborations to expand their assay portfolios and enhance assay sensitivity and specificity. These collaborations aim to advance immunological research and support clinical trial applications. -
Development of Multiplexing and High-Throughput Assay Solutions
The introduction of multiplexing and high-throughput platforms capable of detecting multiple cytokines simultaneously is transforming immune response analysis and driving market adoption.
ELISpot and FluroSpot Assay Market Comapnies
- Abcam Limited
- Bio-Techne Corporation0
- BD
- Cellular Technology Limited
- Mabtech
- Autoimmun Diagnostika GmbH
- Lophius Biosciences GmbH
- Bio-Connect B.V.
- Oxford Immunotec
- U-CyTech
Segments Covered in the Report
By Product
- Assay Kits
- Analyzers
- Ancillary Products
By Application
- Research Applications
- Diagnostics Applications
By End-use
- Hospital and Clinical Labs
- Academic and Research Institutes
- Biopharmaceutical Companies
By Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East & Africa
Ready for more? Dive into the full experience on our website!